Gene-mutation pathway discovery paves way for targeted blood cancers therapies
Approximately 30% of individuals with myeloid malignancy diseases have a mutation in a certain gene called tet methylcytosine dioxygenase 2, or TET2. This gene is responsible for providing instructions on creating certain proteins and is known to have a tumor-suppressive function.

A study published in the September issue of Nature is the first to explain the pathway of TET2’s enzymatic activity that is essential for its tumor-suppressing function. Mingjiang Xu, a molecular medicine professor at the Joe R. and Teresa Lozano Long School of Medicine, the University of Health Science Center at San Antonio , is the co-primary investigator for the study. The work is a collaborative effort between UT Health San Antonio scientists and University of Chicago scientists led by co-primary investigator Chuan He.
“There currently is no specific, targeted treatment or therapy for these TET2-mutated cancers. This pathway opens a door for targeted therapeutics and targeted prevention,” Xu said.
When TET2 is mutated and not working properly, malignant cells can grow out of control. Mutation of this gene was pinpointed years ago as a culprit in blood cancers such as chronic myelomonocytic leukemia, acute myeloid leukemia and myelodysplastic syndromes, but the mechanisms behind how TET2 genetic changes led to disease remained a mystery — until now.
About 15 years ago, Xu was one of the first scientists to discover that the TET2 mutation is sufficient to cause myeloid malignancies in mouse models. His pioneering work led to further research of TET2 and its tumor-suppressing function. After this discovery, dozens of labs around the world sought to understand the underlying mechanisms. However according to Xu, other scientists hit a wall by focusing only on TET2’s effect on DNA.
Looking beyond DNA
His team at the time was the first to show that enzymatic activity of TET2 was essential to mediating tumor-suppressive function. Xu realized that TET2’s effect on DNA was not enough to fully explain the action, and he hypothesized that TET2’s enzymatic effects using RNA as a substrate may be equally important.
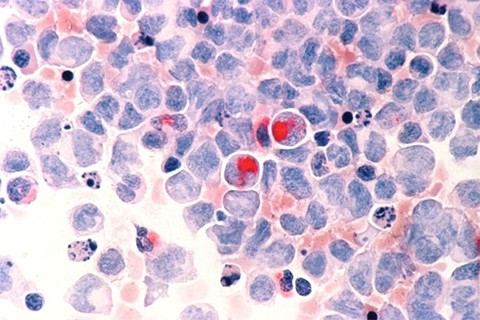
In collaboration with He in Chicago, an expert in RNA modification, they discovered that TET2 can modify chromatin-associated RNA, leading to changes in gene expression. This current study illuminates this process, highlighting some promising new therapeutic targets.
“One thing that surprised us was how powerful this pathway is on TET2-mediated gene expression. It is a quantum structure change mediated by this pathway,” Xu said.
The study showed that the absence of TET2 creates a pathway leading to an open chromatin environment that allows for gene expression changes that can lead to the activation and growth of blood cancer cells.
“We found the MBD6, the reader protein of m⁵C, is a great therapeutic target for treating myeloid malignancies with TET2 mutation,” Xu said.
Next step: Targeted therapies
Xu said that the findings from this study could be transformational in the discovery of treatments and preventive measures for people with TET2 mutation-related diseases. He said the next steps will be working on molecular inhibitors that can disrupt this pathway and lead to highly targeted therapies.
Along with its role in certain blood cancers, about a decade ago it was discovered that about 10% of people aged 70 and older with a genetic mutation (including, but not limited to, TET2 mutation) develop clonal hematopoiesis of indeterminate potential (CHIP).
“These individuals have a significantly higher possibility of developing myeloid cancer and cardiovascular diseases. This makes our findings even more important because the aging population is growing and these individuals need preventive interventions,” Xu said.
Xu said this study is a turning point potentially leading to the exploration of several players within this pathway that could be targets for therapeutic intervention.
According to Xu, this study is also the signature piece of his biomedical career. He was recruited to UT Health San Antonio over five years ago, shortly before receiving National Institutes of Health funding to further explore the effects of TET2 and RNA modification in these diseases. Xu said this discovery was made possible through the trust and support of UT Health San Antonio administration, his team and the supportive environment at the university. This groundbreaking work could not be done without the collaborative efforts of his team members from the departments of Molecular Medicine and Cell Systems and Anatomy, as well as from the University of Chicago team, said Xu.
This article is republished from the UT Health San Antonio Newsroom website. Read the original here.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Adults grow new brain cells
How does the rare birth of these new neurons contribute to cognitive function?
From the journals: JBC
Histone demethylase inhibited by own sequence. MicroRNA reduces cell cycle–related apoptosis. Multipurpose antibiotic takes on staph infections. Read about recent JBC papers on these topics.
Tiny laboratories that fit in your hand can rapidly identify pathogens using electricity
Pathogens have distinct electrical charges, shapes and sizes. Measuring how quickly they move through an electric field can help researchers separate different species in a sample.
Toxoplasma gondii parasite uses unconventional method to make proteins for evasion of drug treatment
This recent study by a team from Bill Sullivan’s lab at the Indiana University School of Medicine was named a Journal of Biological Chemistry Editor’s Pick.

Of genes, chromosomes and oratorios
Jenny Graves has spent her life mapping genes and comparing genomes. Now she’s created a musical opus about evolution of life on this planet — bringing the same drive and experimentalism she brought to the study of marsupial chromosomes.
Ubiquitination by TRIM13: An ingredient contributing to diet-induced atherosclerosis
Researchers help unravel the molecular mechanism behind plaque formation in cardiovascular disease.

